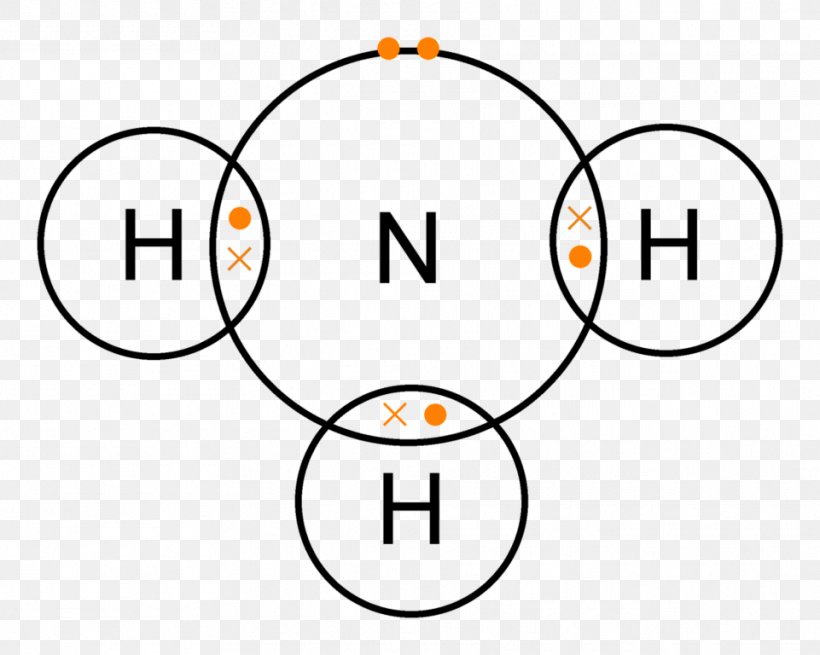
Lewis Structure Ammonia Covalent Bond Lone Pair Chemical Bond, PNG, 961x768px, Lewis Structure
This chemistry video tutorial explains how to draw the lewis structure of NH3 also known as Ammonia.Chemistry - Basic Introduction: https:.
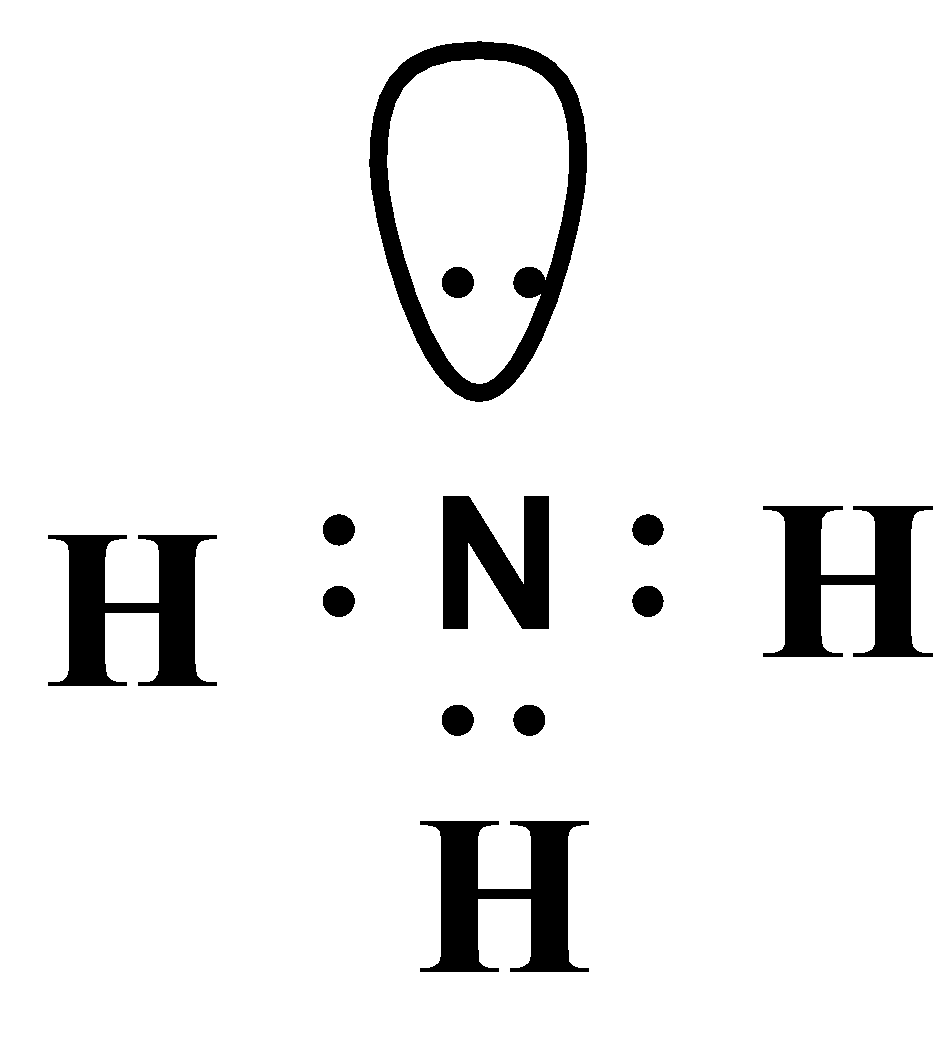
Lewis Dot Diagram Of Ammonia
Ammonia (NH 3) is a commonly tested Lewis structure due to it's widespread use in agriculture as a fertilizer. It also is a good example of a molecule with a trigonal prymidal molecular geometry. There are 8 valence electrons available for the Lewis structure for NH 3. Video: Drawing the Lewis Structure for NH3 It is helpful if you:

Lewis Structure Of Ammonia
1: Structure and Bonding 1.3: Lewis Structures Expand/collapse global location 1.3: Lewis Structures Page ID Using Lewis Dot Symbols to Describe Covalent Bonding

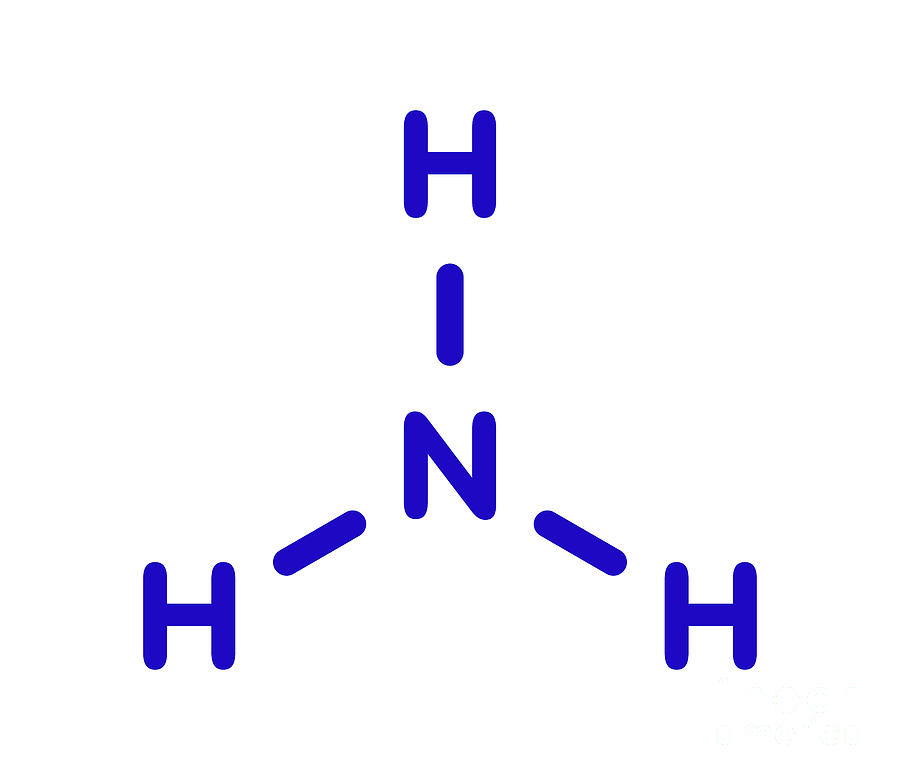
The Shape of the Ammonia Molecule Nh3 Is
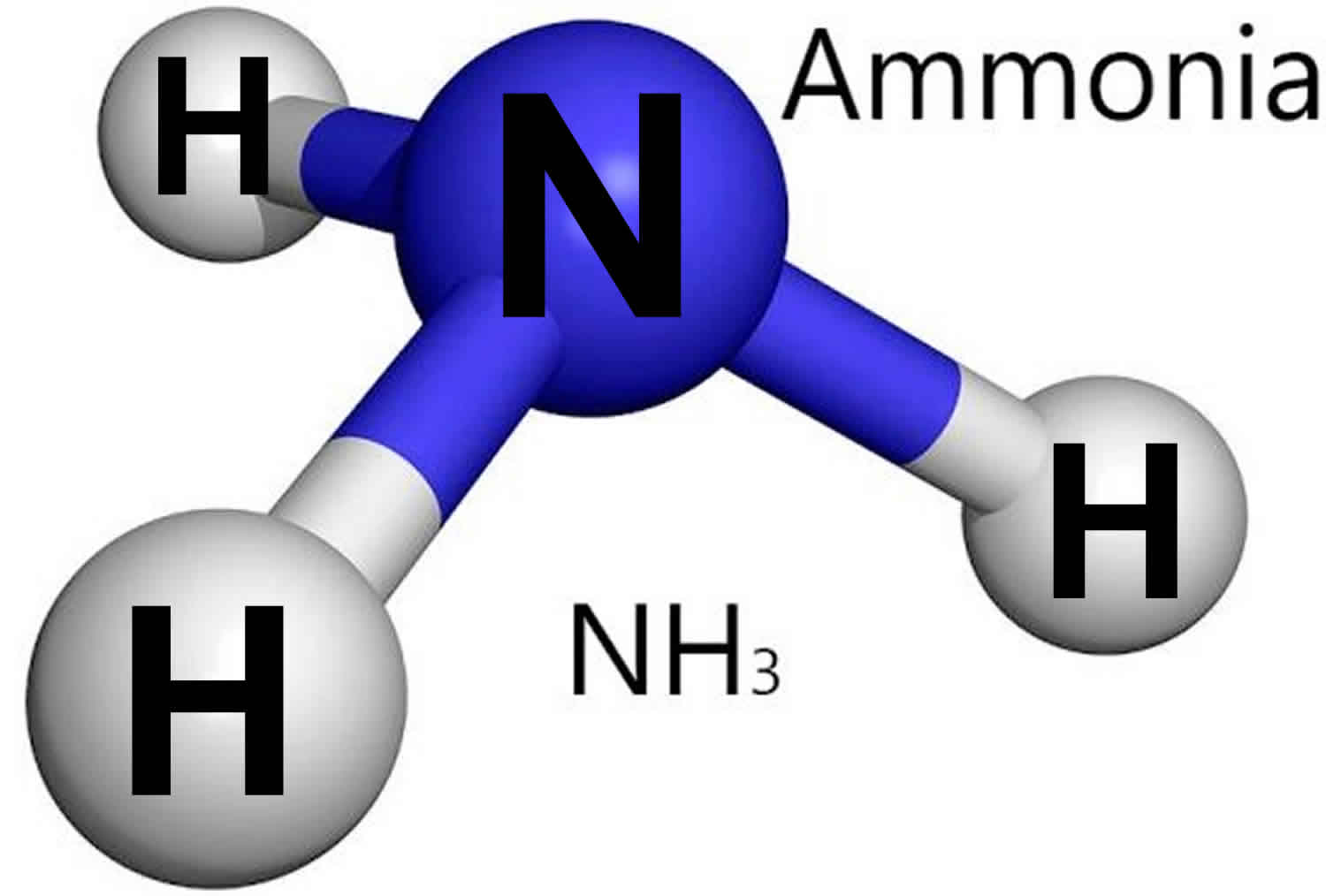
Ammonia is the simplest binary hydride made up of nitrogen and hydrogen denoted by its chemical formulae as NH3. It is a stable pnictogen hydride where all the atoms are covalently bonded to achieve a reactive state. Ammonia is lighter than the air, colorless, and pungent in smell.
How is ammonia represented by an electron dot diagram?
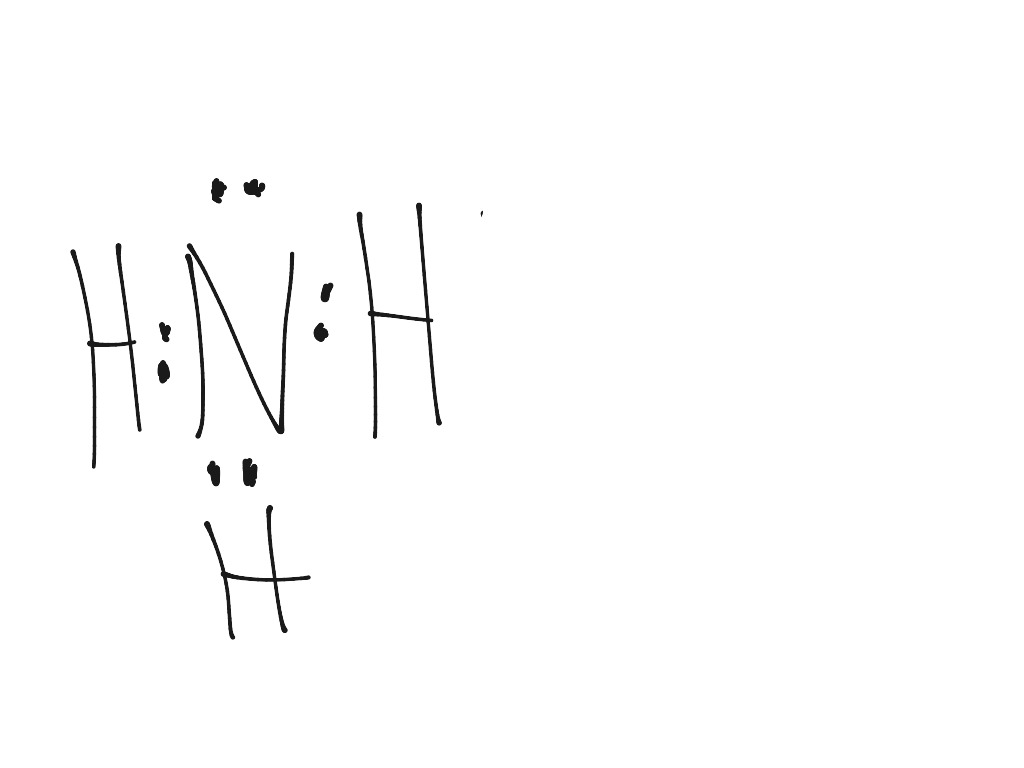
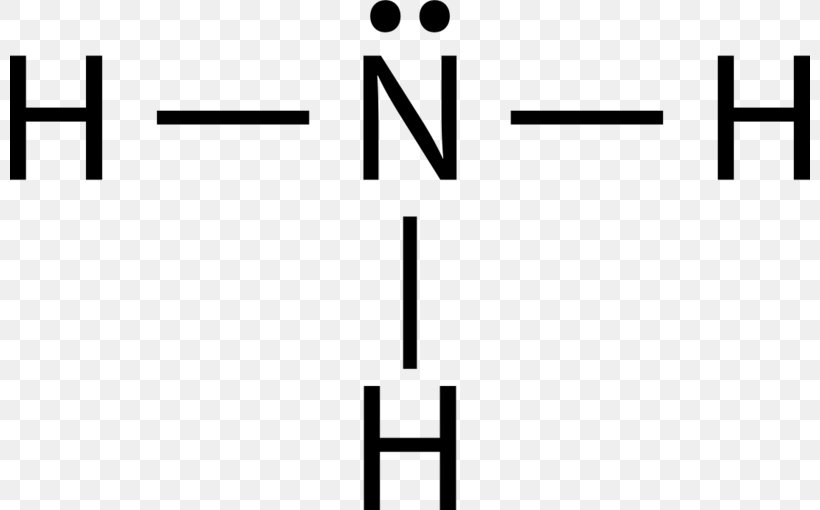
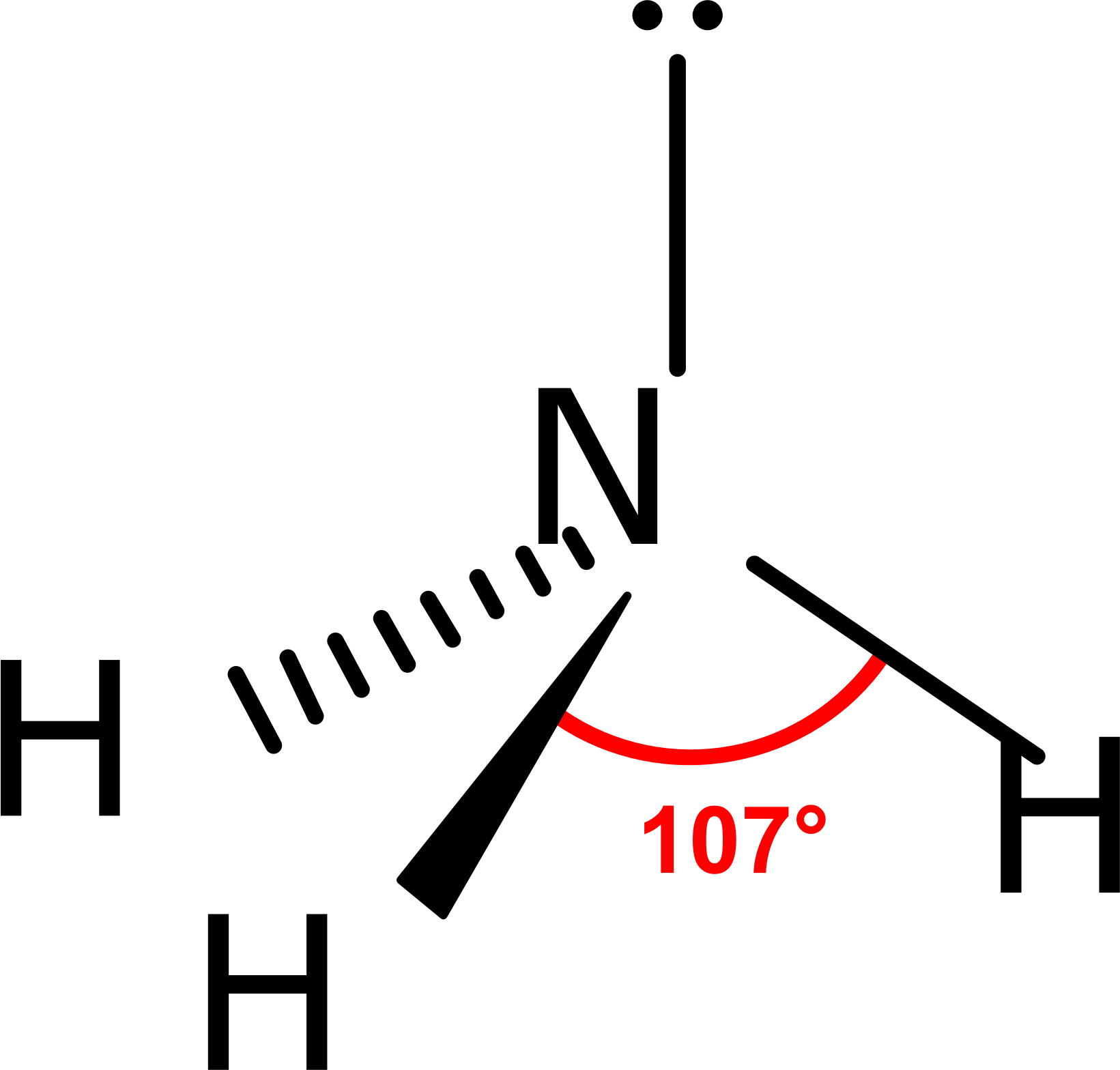
Ammonia (NH3) lewis structure is made up of one nitrogen (N) atom and three hydrogens (H) atoms. In, lewis's structure of NH3, three bond pairs, and one lone pair are present. The nitrogen (N) atom is situated at a central position and the hydrogen (H) atoms are at the outside position in the lewis diagram.
Ammonia Molecule Photograph by Molekuul/science Photo Library Pixels
It consists of hydrogen and nitrogen. In its aqueous form, it is called ammonium hydroxide. This inorganic compound has a pungent smell. In its concentrated form, it is dangerous and caustic. The NH 3 chemical name is ammonia. Table of Contents Ammonia Structure Preparation of Ammonia Properties of Ammonia Uses of Ammonia

Ammonia Formula NH3 Equation, Formation, Structure, Charge
Ammonia (NH 3) Molecule Shape, Geometry, Hybridization. Ammonia lewis structure contains three sigma bonds and one lone pair on nitrogen atom. Therefore, there are total of four electrons regions. So, hybridization of center atom, nitrogen is sp 3.Because there are four electrons regions, geometry is tetrahedral and shape is trigonal pyramidal.
Ammonia Lewis structure Science, Chemistry, Chemical Bonds ShowMe
Learn the steps to draw the Lewis Structure of NH3 (ammonia) in just 1 minute.📌You can draw any lewis structures by following the simple steps mentioned in.
Ammonia, anhydrous ammonia, uses, levels, test & ammonia health effects
Lewis Structures. Page ID. A Lewis Structure is a very simplified representation of the valence shell electrons in a molecule. It is used to show how the electrons are arranged around individual atoms in a molecule. Electrons are shown as "dots" or for bonding electrons as a line between the two atoms. The goal is to obtain the "best" electron.

FileAmmonia lone electron pair 2.svg Wikimedia Commons
Lewis structure of NH3 (ammonia) contains three single bonds between the Nitrogen (N) atom and each Hydrogen (H) atom. The Nitrogen atom (N) is at the center and it is surrounded by 3 Hydrogen atoms (H). The Nitrogen atom has one lone pair. Let's draw and understand this lewis dot structure step by step.

Ammonia Formula Structure, Formula, Properties, Preparation Embibe
NH3 (Ammonia) lewis structure has a Nitrogen atom (N) at the center which is surrounded by three Hydrogen atoms (H). There are 3 single bonds between the Nitrogen atom (N) and each Hydrogen atom (H). There is 1 lone pair on the Nitrogen atom (N).
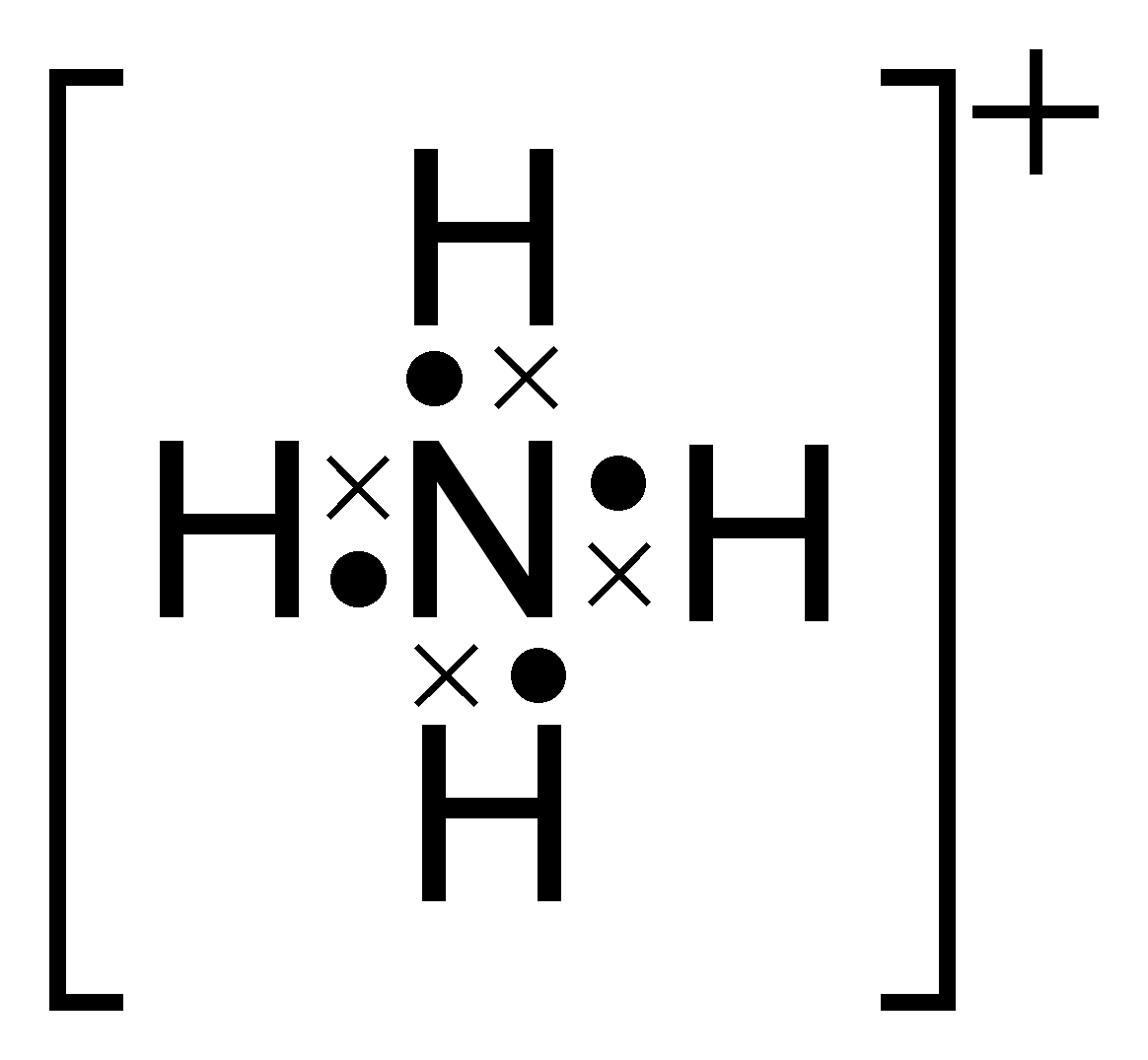
Electron Dot Diagram Of Ammonium Ion
Step 1: Figure out how many electrons the molecule must have, based on the number of valence electrons in each atom. When drawing the structure of an ion, be sure to add/subtract electrons to account for the charge. Step 2: Connect the atoms to each other with single bonds to form a "skeleton structure.".

Estructura de Lewis NH3, Amoniaco » Quimica Online
In the Lewis structure of ammonia, the nitrogen atom forms three single bonds with the hydrogen atoms and has one unshared electron pair. Ammonia Formula. The chemical formula for ammonia is NH3. The formula indicates that there are three hydrogen atoms and one nitrogen atom in each molecule of ammonia. The formula represents the composition of.

Estructura de Lewis NH3, Amoniaco » Quimica Online
Understanding the NH3 Lewis structure is crucial for comprehending the chemical properties and behavior of ammonia. By following the step-by-step guide provided in this article, you can draw the NH3 Lewis structure accurately and gain insights into its molecular bonding.
Lewis Structure Ammonia Lone Pair Ammonium Lewis Pair, PNG, 800x510px, Watercolor, Cartoon
Steps involved in the NH3 Lewis structure: Step 1: Valance Electron Determination: Step 2: Central Metal Atom: Step 3: Connect the Atoms with a lone pair: Step 4: Distribute Lone Pairs: Step 5: Complete the Lewis Structure: Step 6: Formal Charge: Detailed applications: Synthesis/Production:
Clipart Ammonia Lone Electron Pair
A step-by-step explanation of how to draw the NH3 Lewis Dot Structure (Ammonia).For the NH3 structure use the periodic table to find the total number of vale.